Does insulin resistance cause weight gain? The bottom line:
- Insulin resistance and being overweight are both characteristics of type 2 diabetes.
- This guide explores what comes first between insulin resistance and weight gain.
- Understanding the development of the condition allows us to better target treatment.
- Professor Roy Taylor suggests that insulin resistance and weight gain encourage each other in a self-perpetuating cycle.
- Dr Peter Attia firmly believes that insulin resistance drives weight gain and we need to target the cause (insulin resistance) not the effect (obesity).
- Professor Unger highlights the action of glucagon and hyperinsulinemia in the development of type 2 diabetes.
- Dr Malcom Kendrick developed a theory that holds true with the extreme exceptions: the most obese demographic and those with no fat cells.
- Overall, it seems that eating an excessive amount of carbohydrates paired with glucagon action leads to insulin resistance and weight until type 2 diabetes develops.
This question is the basis of an ongoing, intense debate between researchers trying to determine which of the following is more likely:
(A) Insulin resistance causes weight gain.

OR
(B) Weight gain causes insulin resistance.

Since insulin is one of the main hormones responsible for lowering your blood sugar levels, insulin resistance typically means that your blood sugar levels are higher than they should be.
In addition, it also means that you store fat a lot more easily because insulin promotes fat storage.
When blood sugar levels are higher than they should be, but below the diabetic range, it’s known as non-diabetic hyperglycaemia or, more commonly, ‘prediabetes’.
If left untreated, the risk of developing type 2 diabetes, which can have serious complications, is very high.
Answering whether (A) or (B) is more likely helps us understand the development of type 2 diabetes.
If we learn how something develops, then we can find out the most effective target for treatment (insulin resistance and/or weight loss) and, more importantly, prevention.
This in-depth guide will cover 4 potential answers to our question:
- Professor Roy Taylor’s work at Newcastle University
- Dr Peter Attia’s talk on insulin resistance
- Professor Roger Unger’s prize-winning lecture
- Dr Malcolm Kendrick and the extreme exceptions
Medication-assisted weight loss with a future focus
Start with Wegovy or Mounjaro, transition to habit-based health with our support


Current view on the pathway to type 2 diabetes
The commonly held viewpoint on the development of type 2 diabetes is:
- You overeat
- You gain body weight
- You become insulin resistant
- Subsequently, you put on more weight
- This continues until full-blown type 2 diabetes develops
So today, most people believe:
(B)

1. Professor Roy Taylor’s work at Newcastle University
Professor Roy Taylor is a professor of medicine and metabolism at the University of Newcastle. He and his research group have focused on understanding if, and how, you can ‘reverse’ type 2 diabetes.
On the research group’s university website, they have stated that type 2 diabetes can be reversed with major weight loss:
‘It has been possible to work out the basic mechanisms which lead to type 2 diabetes. Too much body fat within the liver and pancreas prevents normal insulin action and prevents normal insulin secretion. Both defects are reversible by substantial weight loss.’
Here’s our first interaction between weight loss and insulin function. Professor Taylor is saying that if you lose weight, you can also return insulin action and secretion to normal.
- Insulin action: refers to insulin sensitivity, i.e. proper insulin action means there’s little or no insulin resistance in the body.
- Insulin secretion: the ability of the beta cells in the pancreas to release insulin into the blood.
The research group goes on to highlight one further critical point. The insulin-secreting function of the pancreas decreases once fat levels go above a person’s individual ‘fat threshold’.
This threshold varies from person to person and is highly associated with genetics and heritage (e.g. people of South Asian origin have a much higher risk and much lower fat thresholds). This is what they say:
‘A crucial point is that individuals have different levels of tolerance of fat within the liver and pancreas. Only when a person has more fat than they can cope with does type 2 diabetes develop. In other words, once a person crosses their personal fat threshold, type 2 diabetes develops. Once they successfully lose weight and go below their personal fat threshold, diabetes will disappear.’
Professor Taylor’s research paper provides an explanation for the whole cycle, which he has called the ‘Twin-cycle hypothesis’.
This cycle is extensively detailed, but this diagram helps to break it down. The cycle is described below, and key points are in bold.
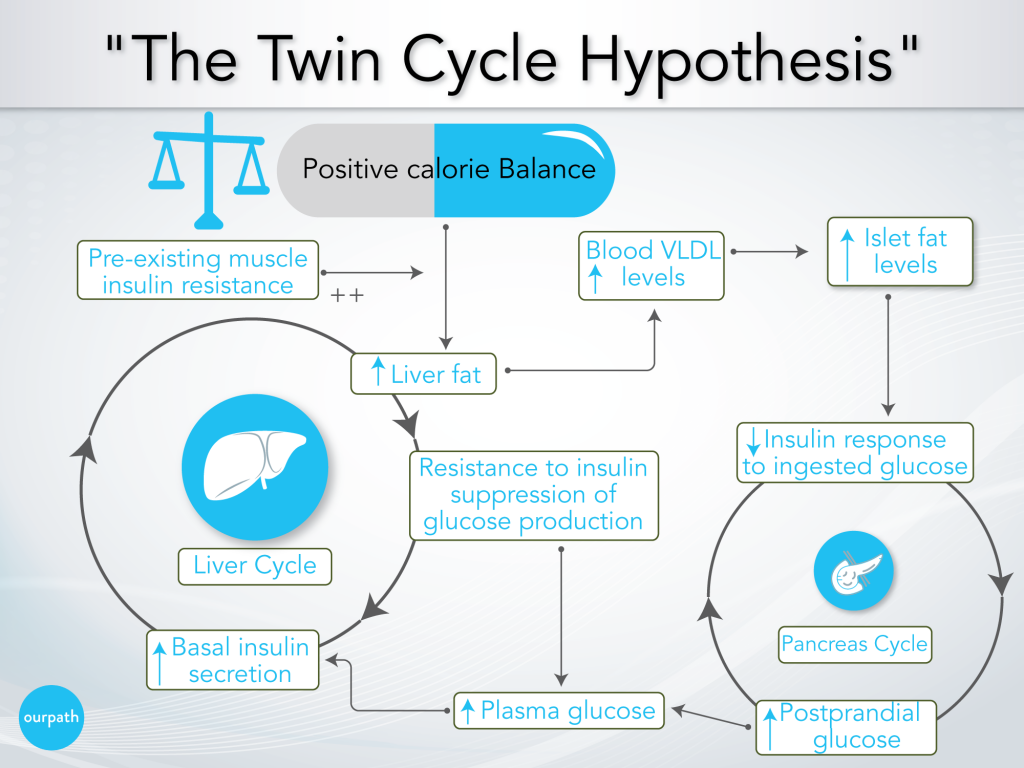
- Over time, a person consistently overeats
- The effect of overeating on the liver
a) Body fat accumulates in the liver, particularly promoted by excess carbohydrate consumption (excess carbohydrates are converted into fat via ‘de novo lipogenesis’), as well as any pre-existing insulin resistance from lifestyle factors or genetics.
b) The liver slowly becomes resistant to insulin, which reduces the suppressing effect of insulin on glucose production in the liver, i.e. the liver will produce and release more glucose into the blood.
c) Over many years, higher fasting blood glucose levels result in higher baseline insulin levels to help reduce blood glucose levels.
d) Elevated insulin levels further promote fat storage in the liver, which becomes a positively reinforcing cycle. - The effect of overeating on the pancreas
a) Meanwhile, increased liver fat leads to increased VLDL export into the blood (a side note: VLDL is the precursor to LDL ‘cholesterol’).
b) Body fat accumulates in the pancreas, which impairs the ability of the pancreatic beta cells to release a significant ‘spike’ of insulin after eating a meal.
c) Following a meal (postprandial), blood glucose levels, therefore, remain high (Professor Taylor says, ‘previous studies have shown [pancreatic] fat stops insulin release’). - Elevated blood glucose levels result in higher insulin secretion over time.
- A more significant insulin release has a knock-on effect on the previously described cycle in the liver (point 2.), which further drives the liver to promote fat production.
- The cycle continues until the system is overwhelmed and full-blown type 2 diabetes develops.
Fundamentally Professor Taylor says that the development of type 2 diabetes/ pre-diabetes is down to overeating and is heightened by eating excess carbohydrates.
Regarding our initial question, what comes first, insulin resistance or weight gain? Professor Taylor’s work suggests that either could come first, and they encourage each other in a self-perpetuating cycle.
Key points:
- Professor Taylor suggests that type 2 diabetes/pre-diabetes is down to overeating.
- Body fat accumulation in the liver and pancreas increases insulin resistance and prevents proper insulin secretion.
- Eating too many carbohydrates, specifically, accelerates this fat accumulation.
- This is a positively reinforcing cycle.
- Weight loss (i.e. fat loss) reverses the overall process.
- The tolerance of fat in the liver and pancreas varies from person to person based on their personal ‘fat threshold’; one person may be able to tolerate a BMI of over 30, while another may not be able to tolerate one over 23.
2. Peter Attia’s TED talk on insulin resistance
Dr Peter Attia is a surgeon from America who developed pre-diabetes many years ago and successfully went into remission. He has dedicated his career to answering our questions and co-founded the Nutrition Science Initiative.
He speaks about his experience and his belief in the relationship between obesity and insulin resistance in his TED talk.
Peter poses the question about whether it’s insulin resistance which is driving obesity and weight gain:
‘Most researchers believe obesity is the cause of insulin resistance. Logically, then, if you want to treat insulin resistance, you get people to lose weight, right? You treat the obesity. But what if we have it backwards? What if obesity isn’t the cause of insulin resistance at all? What if obesity is a coping mechanism for a far more sinister problem going on underneath the cell? I’m not suggesting that obesity is benign, but what I am suggesting is it may be the lesser of two metabolic evils.’
His analogy of banging your shin into a coffee table illustrates the importance of understanding cause vs effect. Banging your shin on a coffee table leaves you with a bruise. The bruise is not the problem but rather a natural response to banging your leg. If we assumed bruises were the problem and targeted the treatment of bruises, we would end up not treating the root cause – people banging their legs on coffee tables. In this analogy:
Bruises = weight gain (the effect)
People banging their shins on coffee tables = insulin resistance (the cause)
So, he’s questioning whether we have the cause and effect of obesity and insulin resistance the wrong way around.
What if insulin resistance causes obesity, and weight gain is merely a natural metabolic response to this ‘underlying epidemic’: insulin resistance?
Peter Attia hypothesises that:
- Over time, consuming high quantities of high-carbohydrate foods slowly builds an underlying level of insulin resistance (even when you’re slim).
- Once this insulin resistance hits a certain threshold, it’s incredibly easy to put on weight.
- This is then when people become overweight/obese with otherwise little to no changes in their diet.
Back to our initial question – what comes first, insulin resistance or weight gain? Peter Attia firmly believes that it’s insulin resistance.

Key points:
- Peter Attia highlights the need to figure out the cause and effect between insulin resistance and weight gain.
- He suggests that high carbohydrate intake builds underlying insulin resistance over time, even in slim individuals.
- Insulin resistance makes it very easy to gain weight.
- This cycle results in obesity.
3. Professor Roger Unger’s prize-winning lecture
An alternative proposal for the development of type 2 diabetes comes from Professor Roger Unger, who has dedicated his career to clarifying ‘the roles of insulin and glucagon in the regulation of normal blood glucose homeostasis and the pathogenesis of diabetes’.
Professor Roger Unger’s 2014 prize-winning lecture turns the whole diabetes debate on its head by providing substantial evidence that it’s glucagon’s action, not insulin, that is crucial to the development of type 2 diabetes (and type 1 diabetes).
Glucagon stimulates the conversion of glycogen in the liver to glucose to be released into the blood.
He states, ‘ Glucagon action is required for type 2 diabetes… the way to stop this disease is to block glucagon action’.
Exploring this in a little more detail:
- In healthy individuals:
- The body releases a significant spike of insulin in reaction to the spike in blood glucose levels after a meal (postprandial).
- This normal spike in insulin is associated with a significant suppression of blood glucagon levels.
- The high insulin and low glucagon levels tell the liver to take away glucose and store this as glycogen.
- In people living with type 2 diabetes:
- The postprandial insulin spike in response to elevated blood glucose levels does not occur.
- Instead, a gradual and sustained rise in insulin levels is seen over time.
- Associated with this, the normal suppression of glucagon levels does not occur.
- These facts combined mean that high levels of glucagon remain in the blood.
- This tells the liver not to store glucose but keep producing it from stored glycogen.
- This is the opposite of what you want to happen directly after consuming a meal that has already elevated your blood glucose levels.
So, why is glucagon not being suppressed in people living with type 2 diabetes after they eat a high-glucose meal?
Professor Unger proposes that it results from insulin resistance in the pancreatic alpha cells. This effect is further compounded by the lack of the postprandial insulin spike in type 2 diabetics.
How do the pancreatic alpha cells become insulin resistant?
Here we return to the common theme running through the work of many of the above research groups: pancreatic fat levels.
We explored earlier Professor Taylor’s proposal that increased pancreatic fat levels impair pancreatic beta cell function. Professor Unger provides a mechanism for this effect:
- Increased fat levels in the pancreas result in an increased accumulation of ceramide (which is toxic) in the pancreas. This has two significant consequences:
- Ceramide kills pancreatic beta cells
- It causes insulin resistance in alpha cells.
This provides a possible explanation for the mechanism for why the postprandial insulin release is suppressed in type 2 diabetics (due to beta cell death), and why glucagon release is not suppressed (alpha cell insulin resistance).
Professor Unger adds that the ‘glucagon receptor is required for dietary obesity and type 2 diabetes’. Studies show that when you remove the glucagon receptor from mice, these mice don’t develop type 2 diabetes or insulin resistance.
Professor Unger hypothesises that:
- Fundamentally, you have to overeat.
- In an average person, this will lead to hyperinsulinemia (excess insulin levels in the blood relative to blood glucose levels).
- Glucagon is an enabler of hyperinsulinemia; without glucagon action, you don’t get hyperinsulinemia.
- Hyperinsulinemia increases ceramide production.
- Ceramide accumulates in excess fatty tissue, including the pancreatic islet cells.
- Ceramide causes beta cell death, suppressing postprandial insulin spikes.
- Ceramide causes alpha cell insulin resistance, preventing insulin’s normal suppression of glucagon.
Back to our initial question – what comes first, insulin resistance or weight gain? Professor Unger believes that it’s overeating (i.e. weight gain) which kickstarts the pathway to type 2 diabetes, but critically that the whole process is enabled by glucagon.
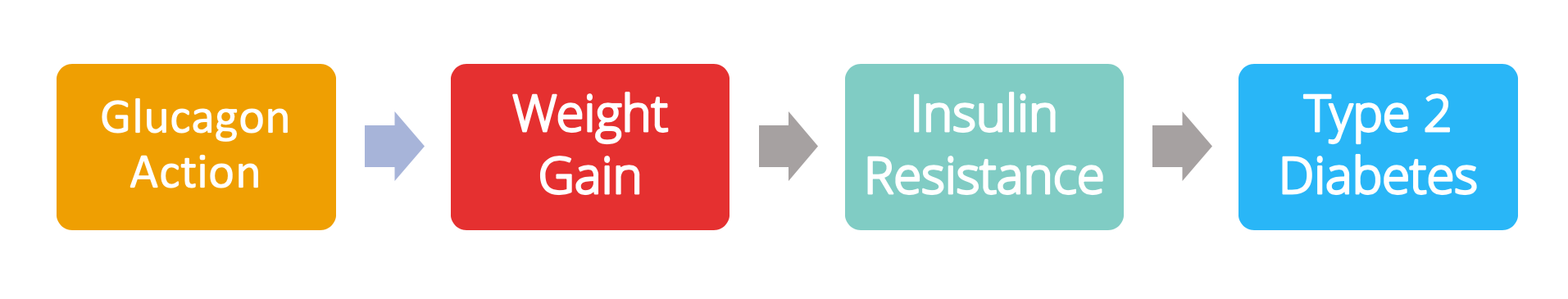
However, he doesn’t clarify the order of steps on this suggested pathway to diabetes. Does weight gain or hyperinsulinemia come first?
Key points:
- Glucagon action does not get suppressed in individuals living with type 2 diabetes as it does in healthy individuals.
- Professor Unger suggests that this is because pancreatic alpha cells become insulin resistant.
- Increased fat in the pancreas results in accumulation of ceramide (toxic), which kills pancreatic beta cells and causes insulin resistance in alpha cells.
- This action suppresses postprandial insulin spikes and prevents the normal suppression of glucagon by insulin resulting in prolonged increased blood glucose levels.
4. Dr Kendrick and the extreme exceptions
There will always be exceptions to any hypothesis. Dr Malcom Kendrick notes that:
‘There is a general principle in science that if you have a proposed mechanism to explain the physiology of a disease, then that mechanism needs to remain true in all scenarios.
Often it’s the case that mechanisms and theories unravel when you look at extreme scenarios.
In this case, this would mean looking at people that have absolutely no fat cells, and the most obese population on the planet.’
What are these exceptions to the mainstream notion of more body fat means a higher risk of diabetes?
- Sumo wrestlers (an extremely obese demographic)
- Berardinelli-Seip lipodystrophy (people with this condition have almost no fat cells)
You might expect that sumo wrestlers would have a high prevalence of type 2 diabetes, and people with Berardinelli-Seip lipodystrophy would have a low prevalence. However, in reality, the opposite is true.
It’s incredibly uncommon for sumo wrestlers to develop type 2 diabetes when training. Conversely, 100% of people with Berardinelli-Seip lipodystrophy have type 2 diabetes.
How is this possible? The hypothesis is that it’s all down to energy storage, i.e. when you eat carbohydrate-containing foods and your blood sugar levels rise, where in your body does that sugar go?
Sumo wrestlers eat high-carb meals, resulting in high insulin levels, which convert excess sugars to fat to be stored.
They don’t become diabetic because extreme physical activity burns up glycogen stores in the liver and muscle, leaving space for insulin to push glucose from the blood into this tissue.
This means there’s no insulin resistance to overcome while sumo wrestlers are training.
Contrastingly, individuals with Berardinelli-Seip lipodystrophy have no fat cells.
So, even if insulin levels dramatically increase, excess energy has nowhere to go after a high-carb meal. This is why insulin resistance develops in this demographic.
Dr Kendrick hypothesises a different mechanism for the development of type 2 diabetes:
- You overeat carbohydrates/sugar.
- You produce too much insulin.
- This forces your body to store fat.
- You become obese.
- At a certain point, insulin resistance develops to block further weight gain.
- This resistance becomes more and more severe until you develop type 2 diabetes.
This model explains the diverse range of people who have type 2 diabetes or are at risk of developing type 2 diabetes.
Dr Kendrick concludes:
‘Whilst those with Beradinelli-Siep lipodystrophy cannot tell us much about diabetes and obesity in ‘normal’ people, this condition does make it very clear that diabetes (insulin resistance, high insulin and high sugar levels) is primarily an issue with energy storage, how the body goes about this storage, and the role that insulin plays.
If there is somewhere for excess energy to go easily, insulin levels will not go up, and nor will blood sugar levels.’
Aside from anything else in this guide, it does provide a compelling rationale for regularly engaging in high-intensity exercise or lifting weights; both deplete your muscle glycogen levels much more effectively than moderate-intensity exercise, like jogging.
Back to our initial question – what comes first, insulin resistance or weight gain? Dr Kendrick believes insulin resistance, or hyperinsulinemia, strongly drives weight gain due to energy storage issues.

Conclusion
Up to this point, this guide has highlighted that type 2 diabetes and prediabetes are much more complex than the commonly held view of you gaining weight and then you get type 2 diabetes.
Weighing up the four answers to the original questions, it’s likely that insulin resistance and/or hyperinsulinemia, coupled with glucagon action, strongly drives weight gain.
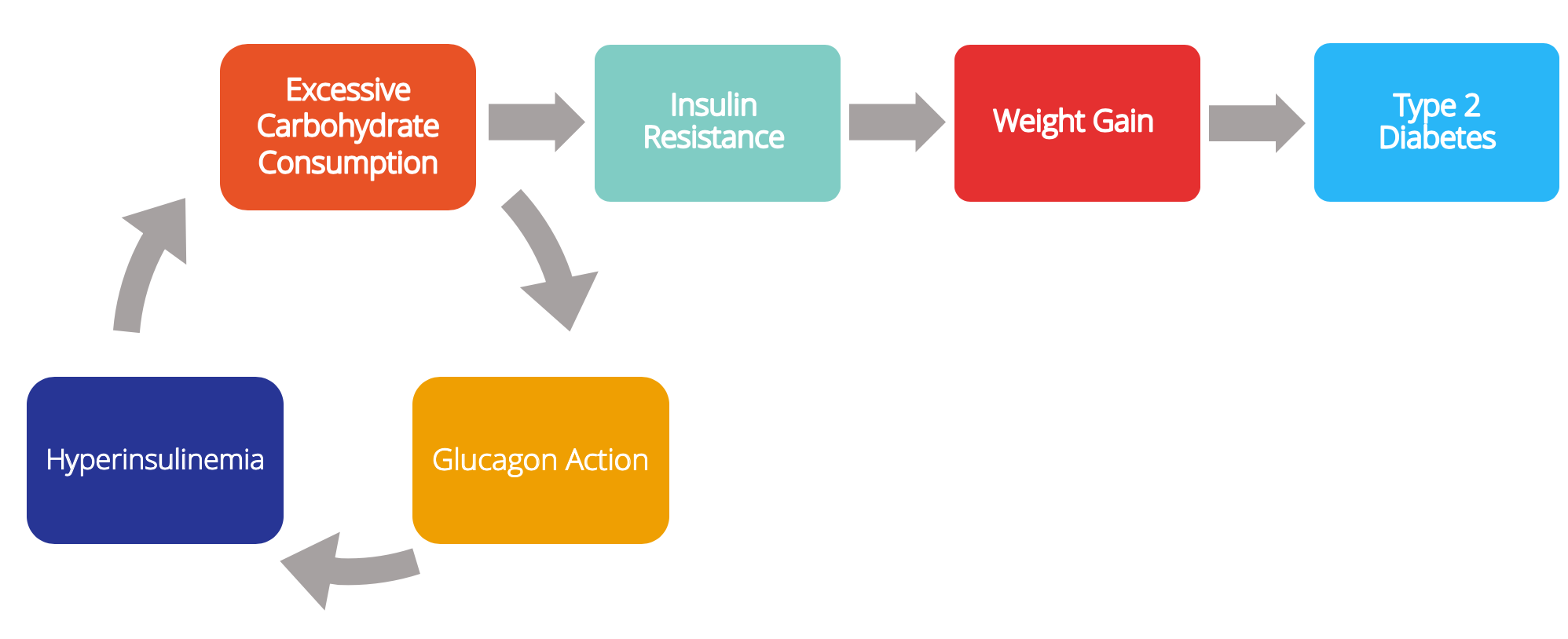
Other factors also appear to be involved in the development of type 2 diabetes, including genetic factors (e.g. personal fat threshold).
Since we cannot change our genes, a lower carbohydrate diet, rather than low-fat, could be an excellent first line of treatment rather than drugs.
Take home message
- Insulin resistance and being overweight are both characteristics of type 2 diabetes.
- This guide explores what comes first between insulin resistance and weight gain.
- Understanding the development of the condition allows us to target treatment better.
- Professor Roy Taylor suggests that insulin resistance and weight gain encourage each other in a self-perpetuating cycle.
- Dr Peter Attia firmly believes that insulin resistance drives weight gain, and we need to target the cause (insulin resistance), not the effect (obesity).
- Professor Unger highlights the action of glucagon and hyperinsulinemia in developing type 2 diabetes.
- Dr Malcom Kendrick developed a theory that holds with extreme exceptions: the most obese demographic and those with no fat cells.
- Overall, it seems that eating an excessive amount of carbohydrates paired with glucagon action leads to insulin resistance and weight until type 2 diabetes develops.


